Coronavirus has been declared as a global pandemic by the World Health Organisation (WHO). Social distancing practices would help to control the spread of infections. Many novel coronavirus studies brought out the necessary implications of the pandemic. Recently, the Coronavirus patient study gave essential insights into the body’s response to the Covid-19 infection. As the novel Covid-19 is drastically spreading its fangs across nations, creating panic among people, now one question remains unanswered. Why is the coronavirus vaccine taking this long to develop even in 2020? There are many world-class labs out there equipped with ultra-modern research equipment. Hence it is a matter of fact for a common man to intrigue upon the delay in developing a vaccine for the pandemic disease. Before we go through vaccine development, it is important to know the process involved in making a vaccine.
In this article, we would like to resolve many of the queries surrounding Covid-19 vaccine development. We will start from the basics and discuss in-depth so that you are much aware of the complex process behind the SARS-CoV-2 vaccine development. Many research institutes across the globe are racing to create a vaccine for Covid-19 infection. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases opined that Finding “a safe and effective vaccine” to prevent infection from the new coronavirus “is an urgent public health priority,”
Coronavirus Vaccine Latest Updates
The first batch of Volunteers receives trail dose of Coronavirus Vaccine
A group of volunteers from the US have become the first to receive the potential vaccine for the Covid-19 virus. In the phase-I study, four patients received the vaccine jab at the Kaiser Permanente Washington health research institute in Seattle, United States. Jennifer, a 43-year old mother of two, has become the first person to receive the coronavirus vaccine. It is interesting to note that given the severity of the Coronavirus outbreak, the human trial bypassed the animal check, which is usually done before approaching humans. The National Institute of Allergy and Infectious Diseases (NIAID) has granted the necessary permission to fast-track the vaccine in humans without testing them in animals.
National Institute of Health (NIH) funds the human trials for the Covid-19 vaccine. The Biotechnology company, Moderna Therapeutics, are making the vaccine. In the following weeks, researchers plan to enroll a total of 45 volunteers the check the efficacy of the vaccine for Coronavirus. The Coronavirus vaccine research across the globe has been happening at a rapid pace and is racing against time.
What are the Phase-1 Clinical Trials?
The initial trials to study the interaction of the vaccine with humans can be considered as the Phase-1 study. During this period, the volunteers would receive vaccine jabs in the upper arm twice with a gap of 28 days. The Phase-1 clinical trial features a small group of healthy volunteers of 60-80 members who will be monitored during the study. They would be under watch for 14 months. During this time, the scientists would observe the interactions of the Covid-19 vaccine with the humans as well as explore any of the side effects that it elicits.

Initially, the coronavirus vaccine volunteers are administered only a low dose of the vaccine, probably 25 micrograms (mcg) per injection. Based on the response of the volunteers to the injection dose, they are given higher doses of upto 250 mcg per injection in the subsequent period. If the vaccine works as designed, then it should elicit an immune response against the surface protein of the novel SARS-Covid-19.
Phase-2, Phase-3 and Phase-4 clinical trials
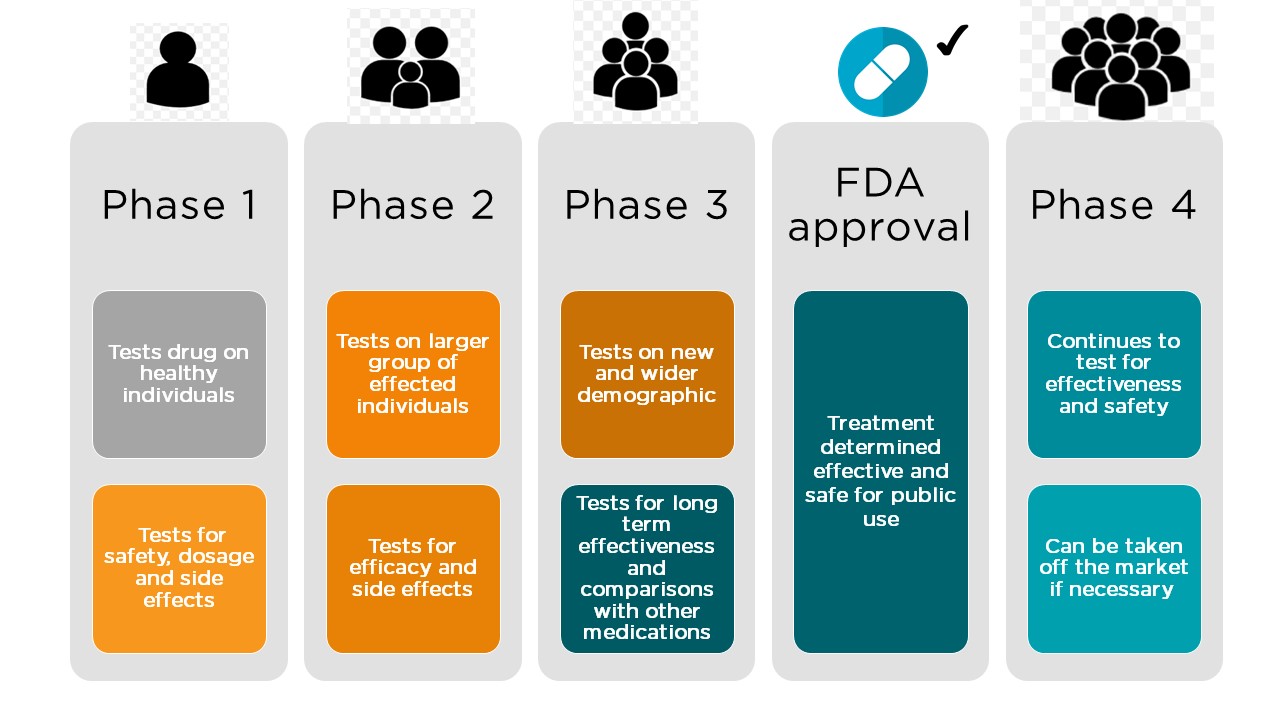
Image: Phases of Drug development
Then subsequently, depending on the success of the vaccine in testing, it would proceed to phase-2 and phase-3 trials that involve larger groups of the human population. The phase-3 trials usually involve around 300-3000 volunteers, and the trials would be conducted for a period spanning one to 4 years. After receiving approval from the Food and Drugs Administration (FDA), the vaccine would then proceed to phase-4 clinical trials, also known as the post-marketing surveillance trials. The researchers then monitor several thousands of volunteers for the potential side-effects. If everything goes well, it may take around 12 to 18 months for the vaccine to be available for widespread use. That is the reason why taking vaccine candidates for regulatory approval involves a large amount of time.
How do Scientists Develop Vaccines?
The method of producing a vaccine is not that easy as it sounds! Most of the experimental vaccines employed involve the use of attenuated/inactivated forms of bacteria that have lost the capacity of virulence. In that sense, the virus or bacteria cannot cause any disease. These inactivated pathogens retain the basic architecture of the bacteria or virus, and hence when exposed to the immune system of a person, it elicits a powerful response. Although the process appears to be smooth, it is nevertheless a bit complicated. For instance, there involves a tremendous investment of time when it comes to isolation and manipulation of bacteria or viruses. Then they should be attenuated to eliminate its virulence. Also, they have to be maintained and produced on a mass scale.

Image: Washington Post
However, most of the viruses have a limited number of proteins and may not elicit an immune response. In such cases, it then involves focussing on the genetic architecture of the viruses/bacteria. It then involves cloning them into a harmless virus/bacteria and allows them to produce a large number of proteins. Even though the large scale production seems to be a challenge, however, it eliminates the steps of isolating the pathogen. The new vaccine against Covid-19 development very well relies on this method.
Also Read: Coronavirus “Close Contact Detection App” alerts Users to Stay Away From Infected Patients
Coronavirus (Covid-19) Vaccine Development
The genome sequence of Coronavirus has already been established. Here one needs to have a basic understanding regarding the production of proteins. In a cell, the genome transcribes into mRNA or the messenger RNA and which then directs the cells to translates them into proteins using the cell machinery. In the current procedure, it involves producing a large number of viral mRNAs and inserting them into the cells. The mRNAs would then be translated inside the cells, and more proteins would be produced. The viral proteins, when exposed to the body’s immune system, elicits a more rigorous response. Everything up to this points looks quite straight forward.

Image: WPTV
In the real scenario, the mRNA molecules are charged, and it is quite challenging for them to pass through the neutral fatty membranes of a cell. To overcome the barrier, the company Moderna involved in coronavirus vaccine development has encapsulated the mRNA molecules inside the tiny pouches of fat to protect them from the surrounding environment. Then the mRNA bags would fuse with the cell membranes, delivering them inside the cells and initiate the production of virus proteins.